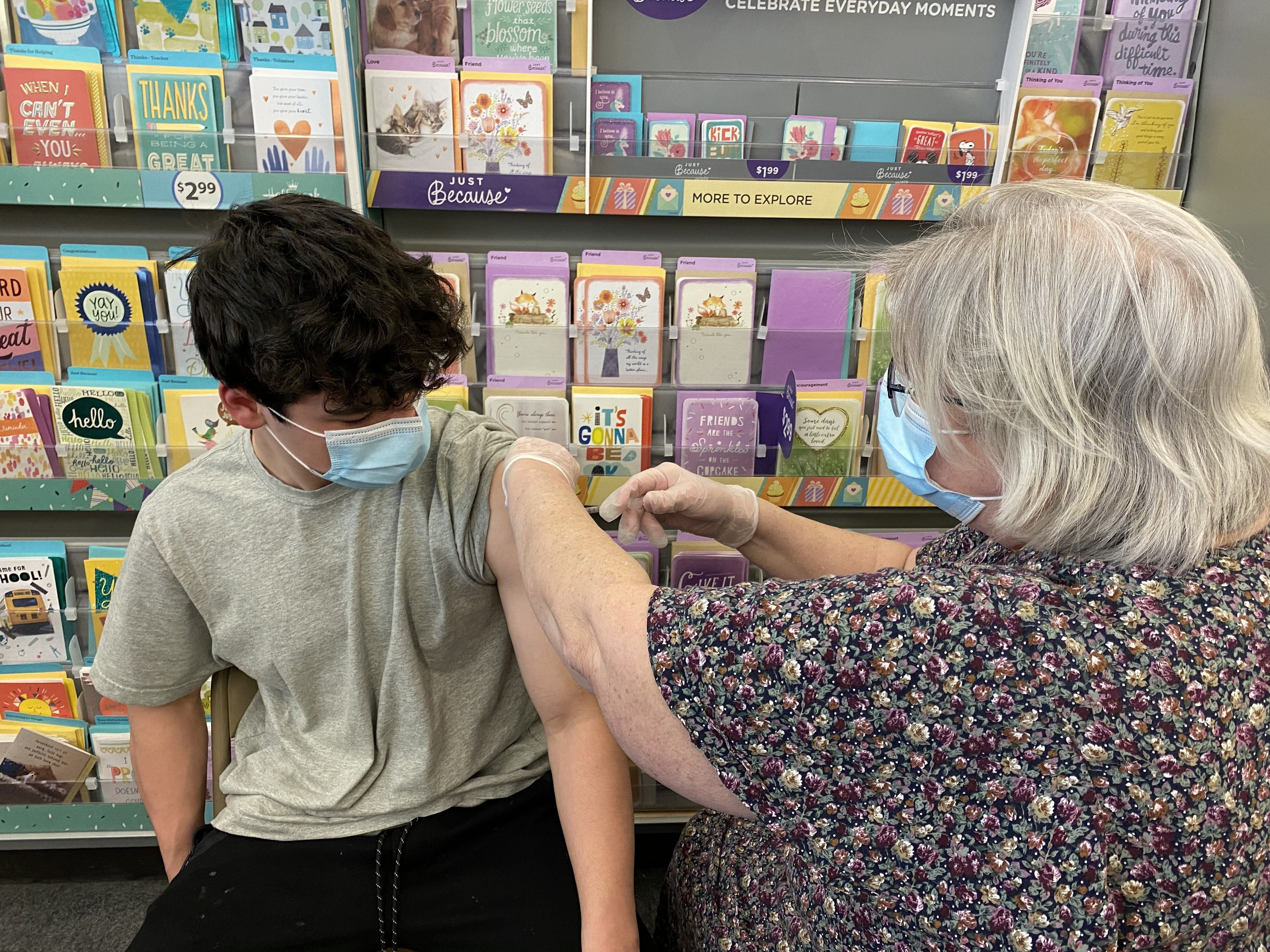
Details of FDA’s emergency use authorization of Pfizer’s mRNA vaccine for children ages 12-15:
Clinical trials of the Pfizer vaccine have included 46,000 subjects:
- This includes 2260 subjects ages 12-15:
- 1131 were given the vaccine, 1005 completed the 2-dose series.
- There were 1129 assigned to placebo arm, 978 completed.
- They were followed for 2 months after completing the 2-dose series.
Results:
- There were 16 cases of COVID 19 among placebo subjects, and no cases among immunized subjects. 100% effective in this group.
- Side effects were mild.
- Only contraindications are severe allergy to any component of the vaccine, primarily polyethylene glycol (list of ingredients contained here).
